NEWS
-
 20202511Press ReleasesGwo Xi Stem Cell Files Drug Master File (DMF) with FDA, Integrating into the Global Regenerative Medicine Industry Chain
20202511Press ReleasesGwo Xi Stem Cell Files Drug Master File (DMF) with FDA, Integrating into the Global Regenerative Medicine Industry ChainGwo Xi Stem Cell’s PIC/S GMP Cell Preparation Factory, located at the Hsinchu Biomedical Science Park in Taiwan, features manufacturing and quality control that meet international standards, serving as a critical foundation for the adipose-derived mesenchymal stem cell (ADSC) starting material bank's successful USFDA DMF registration.
-
 22202510Press ReleasesGwo Xi Stem Cell’s Diabetes Cell Therapy Product GXIPC1® Receives Phase I Trial Results Approval from Vietnam’s MOH
22202510Press ReleasesGwo Xi Stem Cell’s Diabetes Cell Therapy Product GXIPC1® Receives Phase I Trial Results Approval from Vietnam’s MOHGwo Xi Stem Cell’s Diabetes Cell Therapy Product GXIPC1® Receives Phase I Trial Results Approval from Vietnam’s Ministry of Health (MOH) — A Milestone for “Made in Taiwan, Treated Globally”
-
 26202509Press ReleasesGWOXI Stem Cell Secures Taiwan Invention Patent for Stem Cell-Derived Composition in Wound Healing
26202509Press ReleasesGWOXI Stem Cell Secures Taiwan Invention Patent for Stem Cell-Derived Composition in Wound HealingGwo Xi Stem Cell (TPEx: 6704) today announced the grant of a Taiwan invention patent (Patent No. I897580) titled “Pharmaceutical Composition for Wound Repair and Its Preparation Method.” This patented technology leverages Conditioned Medium of Specific Stem Cell-Derived Factors (SpSF-CM), which have demonstrated significant therapeutic effects in promoting wound healing.
-
 09202509Press ReleasesGWOXI Stem Cell Expands Cross-Border Collaborations — Engaging Potential Partners in Thailand
09202509Press ReleasesGWOXI Stem Cell Expands Cross-Border Collaborations — Engaging Potential Partners in ThailandGWOXI Stem Cell showcases its R&D achievements at booth 2R25 during Bio Asia Pacific 2025 in Bangkok, drawing significant attention from international buyers.
-
 22202508Press ReleasesGWOXI Stem Cell Partners with Malaysia’s Cell 101 to Expand into the Asia-Pacific Regenerative Medicine Market
22202508Press ReleasesGWOXI Stem Cell Partners with Malaysia’s Cell 101 to Expand into the Asia-Pacific Regenerative Medicine MarketGWOXI Stem Cell (TPEx: 6704) today announced the signing of a Memorandum of Understanding (MOU) with Malaysia-based Cell 101 International Sdn Bhd, a company mainly focusing on regenerative medicine. Through this collaboration, the two companies aim to jointly expand their presence in the Asia-Pacific regenerative medicine market.
-
 03202507Press ReleasesGWOXI Stem Cell Company Powers Local Regenerative Medicine, Partners with Japan to Open a New Era
03202507Press ReleasesGWOXI Stem Cell Company Powers Local Regenerative Medicine, Partners with Japan to Open a New EraDuring the exhibition, Gwo Xi will show the commodification process of four stem cell therapy products, including the safety and efficacy results in clinical trials, alongside applications of its MSC-derived exosomes. Welcome to join this prestigious international medical event in Japan.
-
 13202505Press ReleasesGWOXI Stem Cell’s GXCPC1® Receives Approval to Initiate Phase III Clinical Trial in Taiwan for Knee Osteoarthritis
13202505Press ReleasesGWOXI Stem Cell’s GXCPC1® Receives Approval to Initiate Phase III Clinical Trial in Taiwan for Knee OsteoarthritisGwo Xi Stem Cell Applied Technology Co., Ltd., (TPEx: 6704) announced yesterday evening that its stem cell medicine “GXCPC1®”, an allogeneic adipose-derived mesenchymal for the treatment of knee osteoarthritis, has received Phase III clinical trial approval from Taiwan Food and Drug Administration (TFDA) to initiate a Phase III clinical trial. This approval represents an important milestone for the advancement of GXCPC1®.
-
 22202504Press ReleasesGWOXI Stem Cell Seizes Opportunities under Taiwan’s Dual Regenerative Medicine Acts with a Dual-Track Strategy in Stem Cell Ther
22202504Press ReleasesGWOXI Stem Cell Seizes Opportunities under Taiwan’s Dual Regenerative Medicine Acts with a Dual-Track Strategy in Stem Cell TherGWOXI Stem Cell is actively positioning to capture emerging market opportunities by strengthening its intellectual property portfolio, progressing clinical trials of its cell therapy medicines, and enhancing the manufacturing capabilities while applying PIC/S GMP certification, which is also expected to obtain the certification by the end of 2025.
-
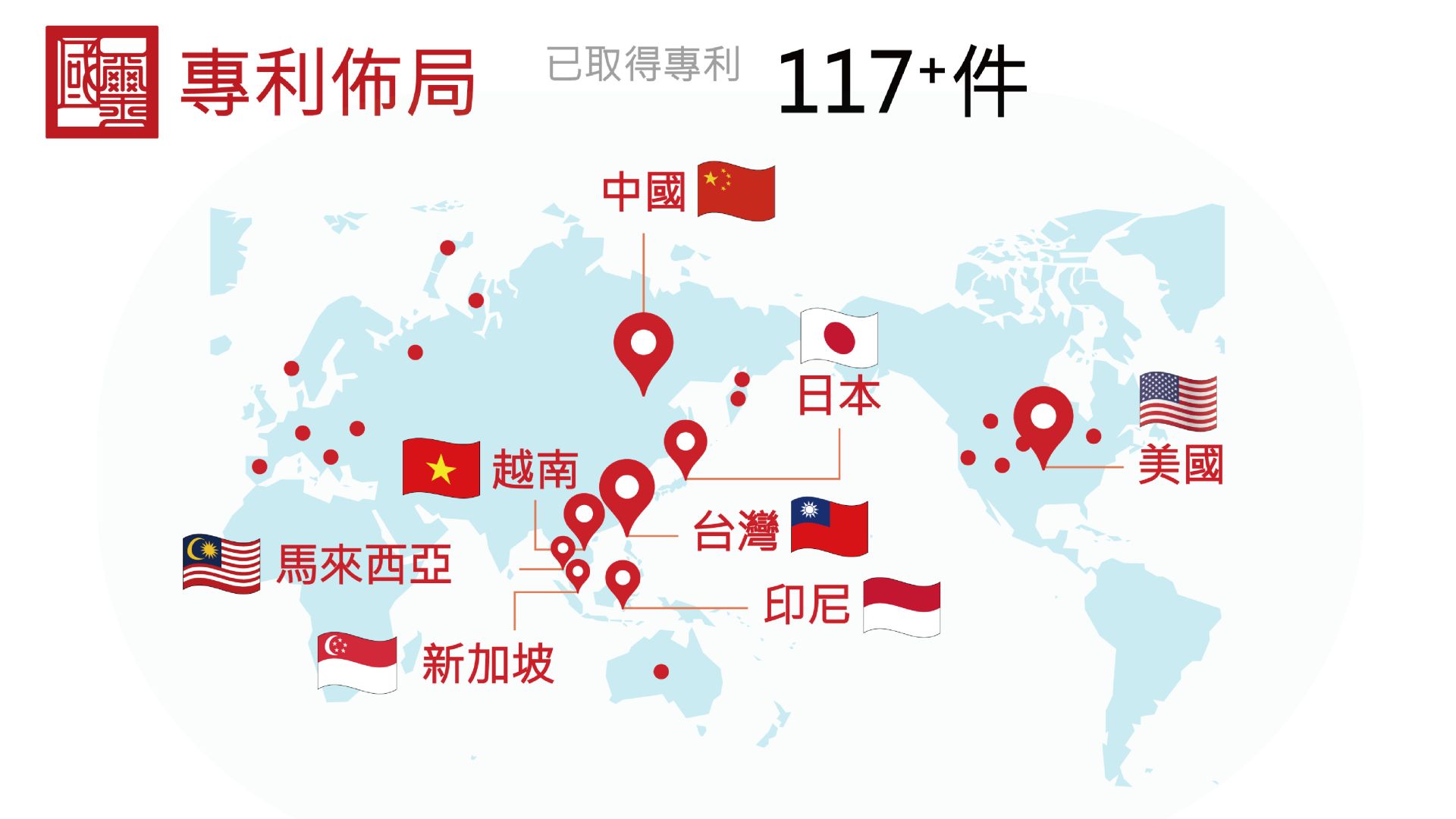 15202504Press ReleasesGWOXI Secures More Than 117 Stem Cell Technology Patents to Drive Global Market Expansion.
15202504Press ReleasesGWOXI Secures More Than 117 Stem Cell Technology Patents to Drive Global Market Expansion.Leveraging its robust patent portfolio and integrated technology capabilities across industry chain, GWOXI (TPEx.: 6704) has successfully advanced multiple innovative technologies from preclinical studies to clinical trials, offering novel therapeutic options for patients.
-
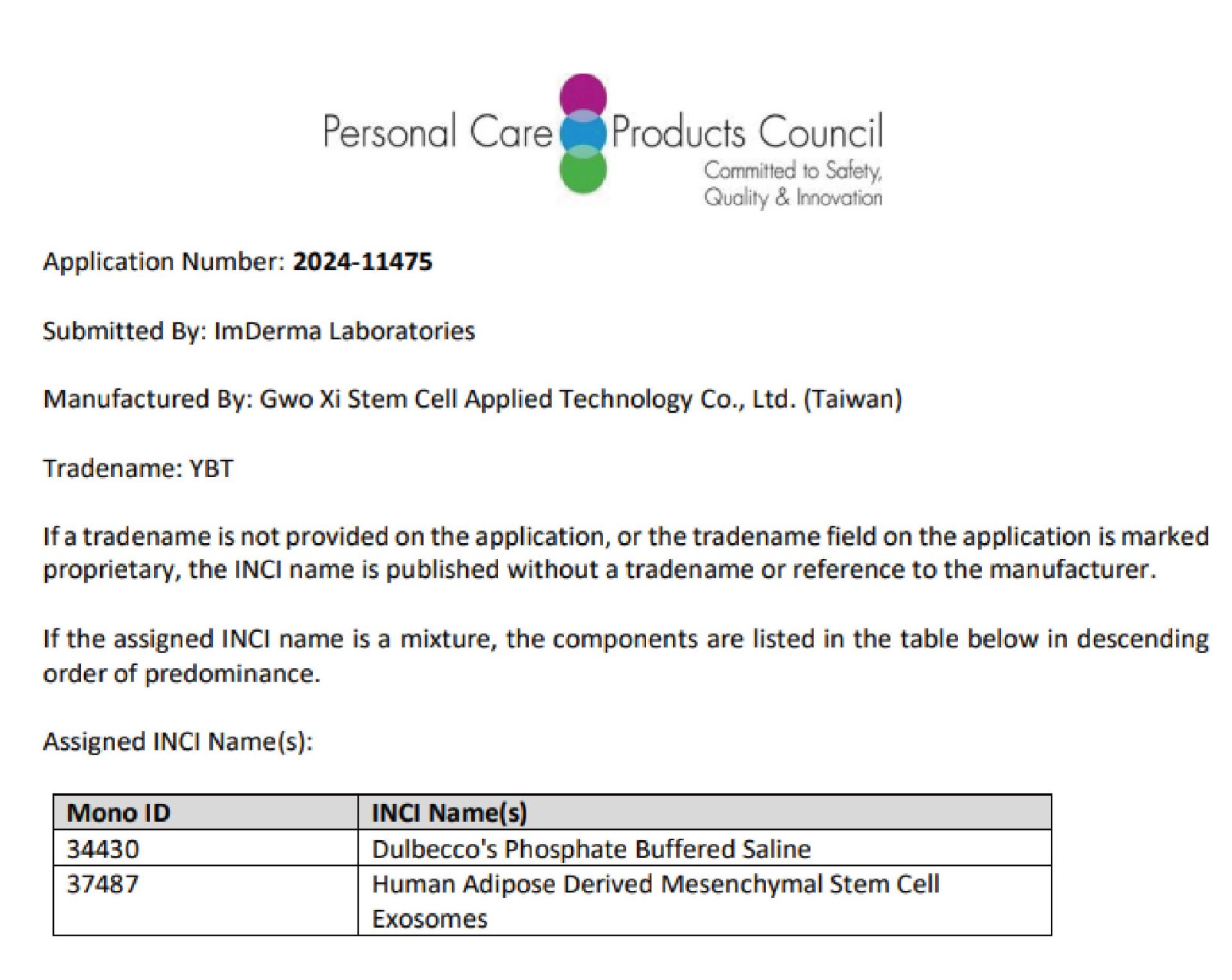 20202503Press ReleasesGWOXI Stem Cell Receives INCI Certification for Human Adipose-Derived Mesenchymal Stem Cell Exosomes
20202503Press ReleasesGWOXI Stem Cell Receives INCI Certification for Human Adipose-Derived Mesenchymal Stem Cell ExosomesGwo Xi Stem Cell’s “Human Adipose-Derived Mesenchymal Stem Cell Exosomes” have obtained INCI name certification as a new cosmetic ingredient.
