GWOXI Stem Cell Expands Cross-Border Collaborations — Engaging Potential Partners in Thailand
GWOXI Stem Cell (TPEx: 6704) continues to expand its strategic partnerships across the Asia-Pacific Region. Following its participation in the Regenerative Medicine Expo Japan, the company recently attended Bio Asia Pacific 2025 in Bangkok, Thailand, one of the region’s largest biotechnology conventions. Invited by the Taiwan External Trade Development Council (TAITRA), GWOXI joined other leading Taiwanese pharmaceutical and biomedical companies in the Taiwan Healthcare Pavilion to showcase Taiwan’s advanced technologies in the field of cell therapy. Moreover, GWOXI was invited by the organizers to present at the biotech forum, where it highlighted its latest advancement in stem cell new drug development, exosome technologies, and CDMO services, drawing strong interest from participants. These initiatives reflect GWOXI’s commitment to advancing its regenerative medicine in Southeast Asia and strengthening international collaboration and market engagement.

GWOXI Stem Cell showcases its R&D achievements at booth 2R25 during Bio Asia Pacific 2025 in Bangkok, drawing significant attention from international buyers.
Leveraging its advanced stem cell-based technologies and cell therapy products developed in Taiwan, GWOXI continues to advance its product pipeline. GXCPC1®, an allogeneic stem cell medicine for knee osteoarthritis, has entered Phase III clinical trials, demonstrating both safety and efficacy, underscoring the company’s strong capability in consistently producing high-quality stem cell medicines. Meanwhile, GXNPC1®, an autologous stem cell medicine for chronic ischemic stroke, has entered the Phase III application stage and is expected to obtain a conditional approval under Taiwan’s “Dual Regenerative Acts,” positioning GXNPC1® could become the first approved stem cell therapy product in Taiwan. Both investigational products are derived from human adipose-derived mesenchymal stem cells (hADSCs) and enhanced with GWOXI’s proprietary core technology, Nigiro-Med®, which exerts designated therapeutic effects for each drug through immunomodulation, homing effect, and paracrine effect. These products have demonstrated favorable safety and preliminary efficacy, providing new therapeutic options for patients suffering stroke and osteoarthritis worldwide. During the exhibition in Thailand, GWOXI’s clinical achievements attracted interests from multiple local hospitals and companies. Numerous business matchmaking meetings were held throughout the event, and the company is now preparing Memoranda of Understanding (MOUs) and Non-Disclosure Agreements (NDAs) with potential partners in Thailand, laying the groundwork for future collaborations.
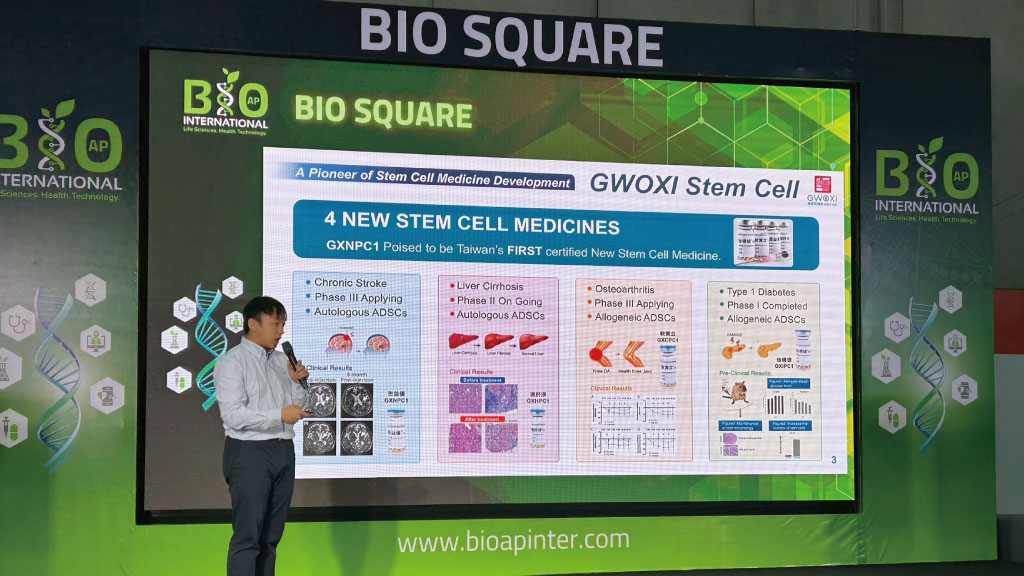
GWOXI’s stem cell therapy products for chronic stroke and knee osteoarthritis are currently in Phase III clinical trials, highlighting the company’s advanced progress in cell therapy industry worldwide.
Exosome Applications in Aesthetic Medicine and International Collaboration
In addition to stem cell therapy, GWOXI has also been actively developing its exosome platform in recent years. Its human adipose-derived mesenchymal stem cell exosomes have obtained INCI name certification (INCI Name: Human Adipose-Derived MSC Exosome, Mono ID: 37487). Adopting its pharmaceutical-grade processes, GWOXI produces MSC-derived exosomes with high purity and batch-to-batch consistency, supporting a variety of applications in aesthetic, beauty product, and medical field. At Bio Asia Pacific 2025 in Bangkok, GWOXI engaged with industry partners to promote its CDMO services, further strengthening collaborations across Southeast Asia and driving diversified cross-border business models.
Southeast Asia: A High-Potential Market
GWOXI Stem Cell Chairman Dr. Ming-Hsi Chuang stated: “Thailand has a well-developed national healthcare system and a thriving self-pay and medical tourism market. Precision and preventive medicine are rapidly emerging within its high-end medical sector. The regenerative medicine and cell therapy markets are expected to grow rapidly in Thailand. As a medical hub in Asia, Thailand has been actively enhancing its regulatory framework, clinical research environment, and healthcare infrastructure—making it an ideal gateway for introducing innovative medical products.” Through information exchange with local institutions, GWOXI aims to establish collaborations with healthcare provider and biopharmaceutical companies across various models—including distribution, licensing transaction, and CDMO service partnership—in order to build long-term, stable business operations in the Southeast Asian market.

GWOXI’s pharmaceutical-grade exosome raw materials are entering the high-end aesthetic and medical markets in Southeast Asia, while actively promoting cross-border collaborations.
International-Standard Manufacturing to Support Cross-Border Clinical Development
GWOXI operates a PIC/S GMP stem cell preparation factory and possesses full capabilities for cross-border transportation of stem cell-based products. The company produces mesenchymal stem cells (MSCs) and MSC-derived exosome as raw materials that meet international clinical standards, and offers a broad range of collaboration models, including CDMO/CMO services, access to a qualified stem cell bank for use as starting material in clinical therapies, and stem cell product supply—enabling overseas healthcare institutions and enterprises to rapidly adopt regenerative therapies. Through advanced manufacturing and rigorous quality control, GWOXI aims to set a benchmark for regenerative medicinal products and become a reliable supplier in the ASEAN market.
