Approved Technology
Regenerative medicine is becoming increasingly prominent. In the past, cell therapy could only be performed through clinical trials, but now the Ministry of Health and Welfare (MOHW) has regulated several cell therapies under the "Regulations for the Administration of Specific Medical Examination and Medical Instrument Application."
This framework lists six types of cell therapy corresponding to 13 indications, with the option for applications beyond those specified. Through the joint efforts of medical institutions and cell therapy companies, regenerative medicine is no longer just a slogan but a practical, implementable option for the public, which will significantly improve the quality of healthcare for the Taiwanese people.
| Cell Types | Indications | Other Matters |
| Autologous CD34+ selection Peripheral blood stem cell therapy |
1. Chronic ischemic stroke 2. Critical limb ischemia |
1. In accordance with Appendix 3 of the latest Special Regulations or as announced in relevant regulations. 2. Clinics are eligible to apply only after they have passed a relevant evaluation or accreditation conducted by the competent authority or a professional institution commissioned by it. |
| Autologous immune cell therapy | 1. Hematological malignancies unresponsive to standard treatment 2. Stage I to III solid tumors unresponsive to standard treatment 3. Stage IV solid tumor |
|
| Autologous adipose stem cell therapy | 1. Chronic or difficult-to-heal wounds that have not healed for six weeks or more. 2. Extensive burns or traumatic skin injuries covering more than 20% of the total body surface area (TBSA). 3. Subcutaneous and soft tissue defects 4. Degenerative arthritis and knee joint cartilage defects |
|
| Autologous fibroblast therapy/td> | Skin defects: Filling and repair of wrinkles, indentations, and scars |
|
| Autologous bone marrow mesenchymal stem cell therapy | 1. Degenerative arthritis and knee joint cartilage defects 2. Spinal cord injury |
|
| Autologous chondrocyte therapy | Knee joint cartilage defects |
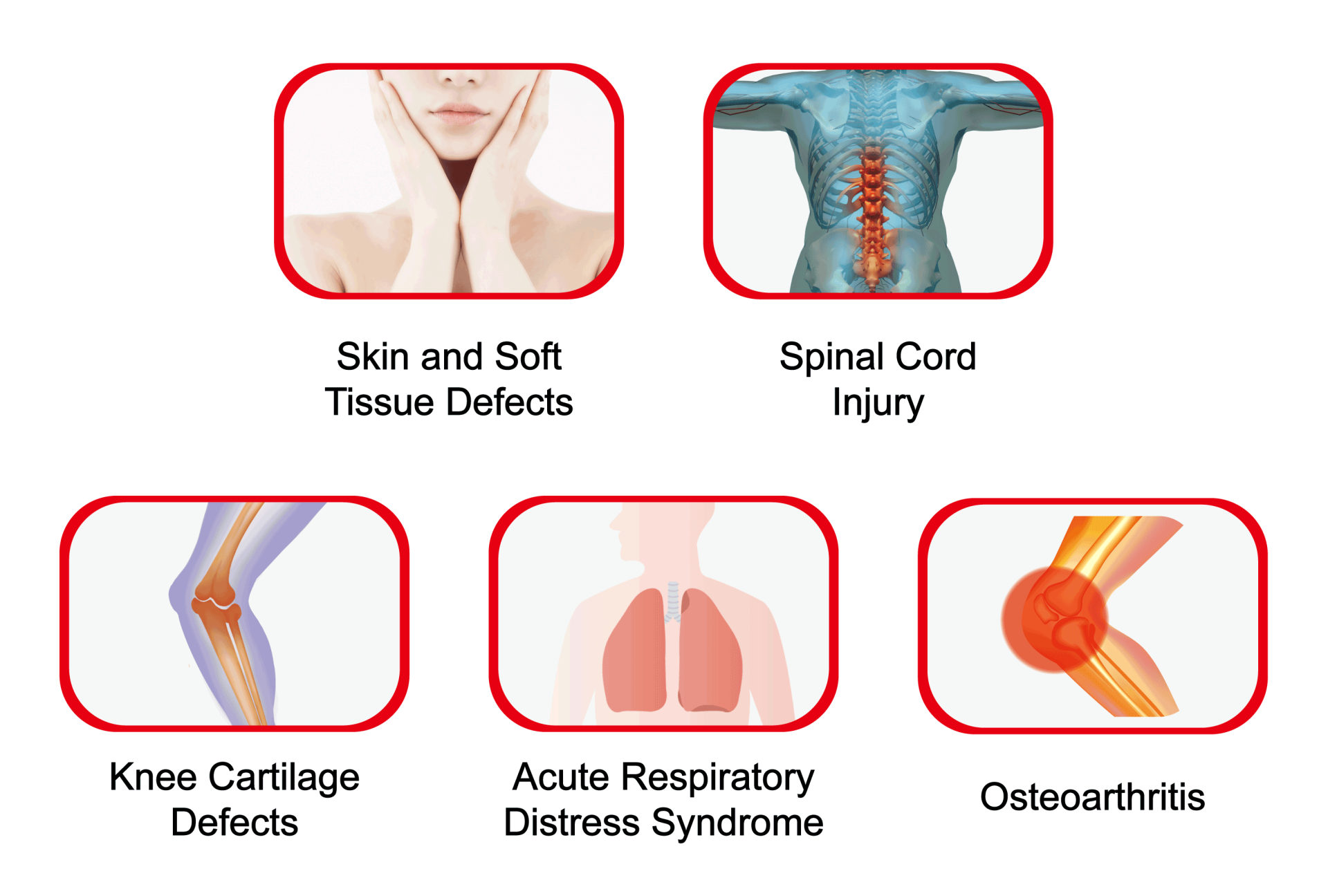
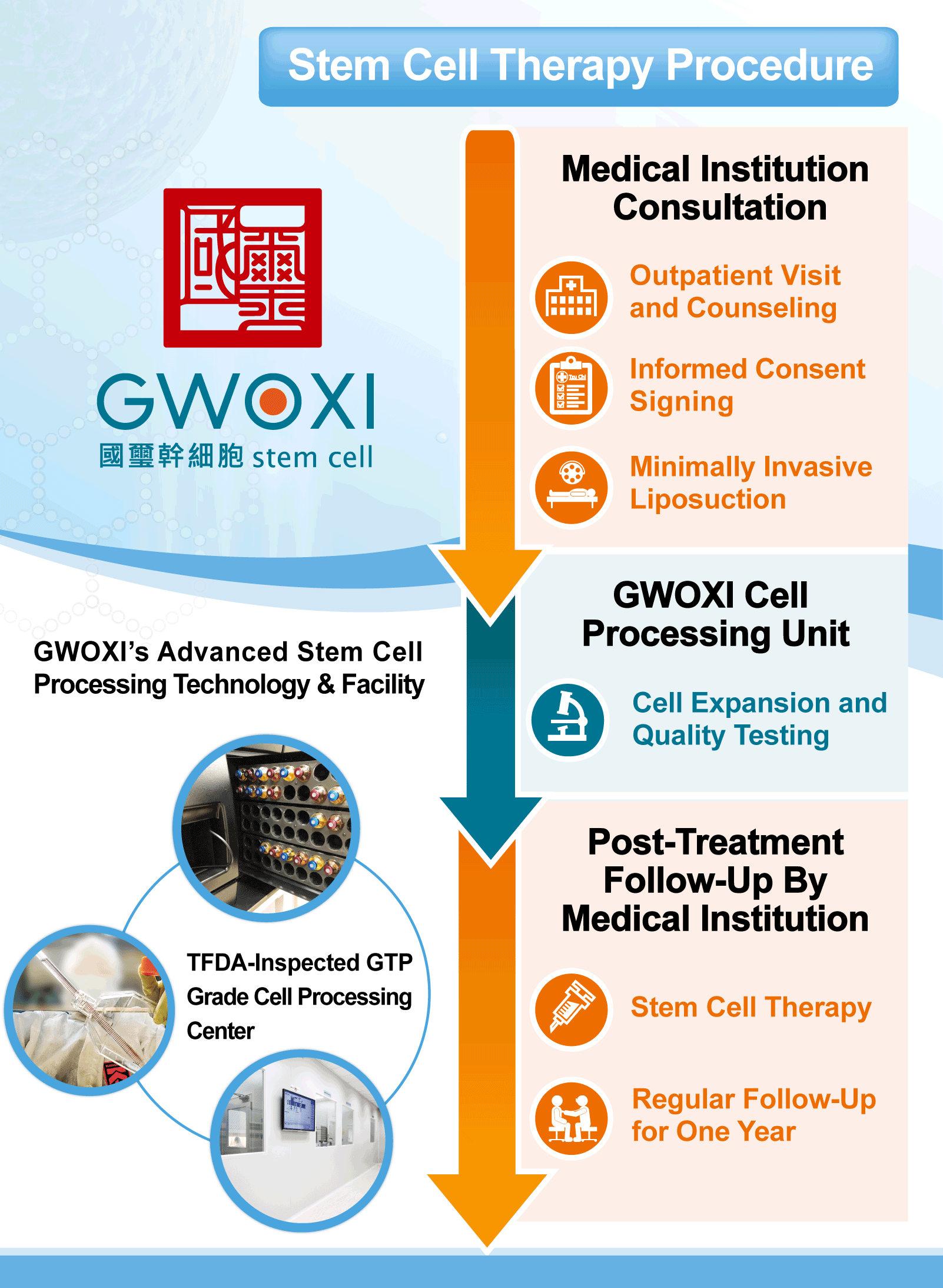
GWOXI Stem Cell Technology has been a long-standing leader in stem cell R&D and therapeutic applications. The company’s journey began with compassionate use collaborations with medical centers for conditions like stroke and cerebellar atrophy. Following the introduction of the Special Regulations, GWOXI has become even more proactive, partnering with numerous medical institutions. The submitted applications for approval include using stem cells to treat subcutaneous and soft tissue defects, spinal cord injury, chronic ischemic stroke, knee cartilage defects, and acute respiratory distress, among others. By putting its many years of experience into these therapeutic applications, GWOXI aims to work with its partners to raise Taiwan’s medical standards.
In a partnership with Hualien Tzu Chi Hospital, we offer the only treatment in Taiwan that uses autologous adipose stem cells for subcutaneous and soft tissue defects.After a professional evaluation by a physician, the patient undergoes subcutaneous filling with their own adipose stem cells. The primary benefits are a long-lasting effect, a natural appearance, and a short recovery period. We hope to give patients renewed confidence and a brand new life.
In partnership with the Kaohsiung Armed Forces General Hospital, we are using autologous bone marrow mesenchymal stem cells to treat spinal cord injury.We hope that this cell therapy will improve the patient's lower limb sensation and urinary functions. The ultimate goal is to help patients regain lower limb motor function, which will improve their self-care ability and restore their quality of life.
Starting in 2021, plastic surgery, aesthetic medicine, and dermatology clinics that have received cell therapy quality certification became eligible to apply for designated cell therapy procedures, such as autologous adipose stem cell therapy for subcutaneous and soft tissue defects. To support this expansion, GWOXI Stem Cell Technology established guidance mechanisms and documentation to help clinics pass their certification. This initiative brings high-quality stem cell therapy into broader clinical use, extending these options to every corner of Taiwan. In addition to the aforementioned items, GWOXI continues its dedicated work with multiple hospitals on various other indications, including knee cartilage defects and chronic ischemic stroke. We hope to benefit more patients in the future by offering a more diverse range of cell therapy options and providing comprehensive, high-quality healthcare.
Press Releases
Press Releases-
 20202511Press ReleasesGwo Xi Stem Cell Files Drug Master File (DMF) with FDA, Integrating into the Global Regenerative Medicine Industry Chain
20202511Press ReleasesGwo Xi Stem Cell Files Drug Master File (DMF) with FDA, Integrating into the Global Regenerative Medicine Industry Chain -
 22202510Press ReleasesGwo Xi Stem Cell’s Diabetes Cell Therapy Product GXIPC1® Receives Phase I Trial Results Approval from Vietnam’s MOH
22202510Press ReleasesGwo Xi Stem Cell’s Diabetes Cell Therapy Product GXIPC1® Receives Phase I Trial Results Approval from Vietnam’s MOH -
 26202509Press ReleasesGWOXI Stem Cell Secures Taiwan Invention Patent for Stem Cell-Derived Composition in Wound Healing
26202509Press ReleasesGWOXI Stem Cell Secures Taiwan Invention Patent for Stem Cell-Derived Composition in Wound Healing
