Exosome CDMO
Exosome CDMO
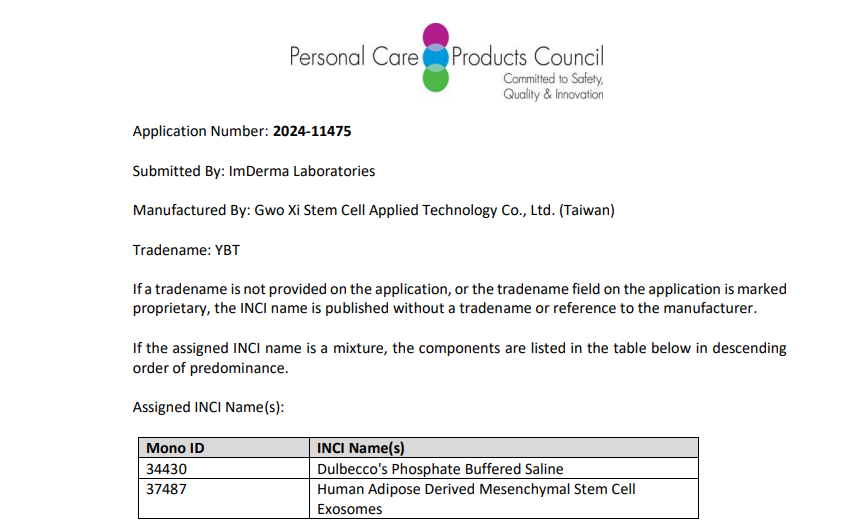
GWOXI is committed to producing gold standard of pharmaceutical grade stem cell-derived exosomes. Our platform can produce pharmaceutical grade exosomes derived from a qualified stem cell source, e.g., hADSC, hWJMSC, hBMSC, sheep-WJMSC.
Platform description:
We use exclusively patented technologies to empower stem cells to secrete functional exosomes. After going through a strict isolation and purification processes, we conduct comprehensive testing to characterize the extracted exosome, ensuring its quality.
The testing processes include:
- 1. Nanoparticle Tracking Analysis (NTA): Accurate analysis for detecting exosome size and concentration.
- 2. Biomarker analysis: Confirm the exosomes’identity.
- 3. Protein concentration analysis: Calculate the protein content in the final exosome formulation.
- 4. Growth factor assay: Verify growth factor levels in exosomes.
- 5. Stem cell characterization: Ensure the quality of stem cell sources.
- 6. Microorganism testing: Ensure the safety of the final exosome formulation.
Quality Assurance:
Our strict quality control ensures the stability and purity of exosomes. GWOXI Stem Cell Exosomes, integrating cutting-edge technology and strict quality control, is your reliable partner in the field of regenerative medicine.
✅ Certified to meet international standards for cosmetic ingredients.(INCI Name)
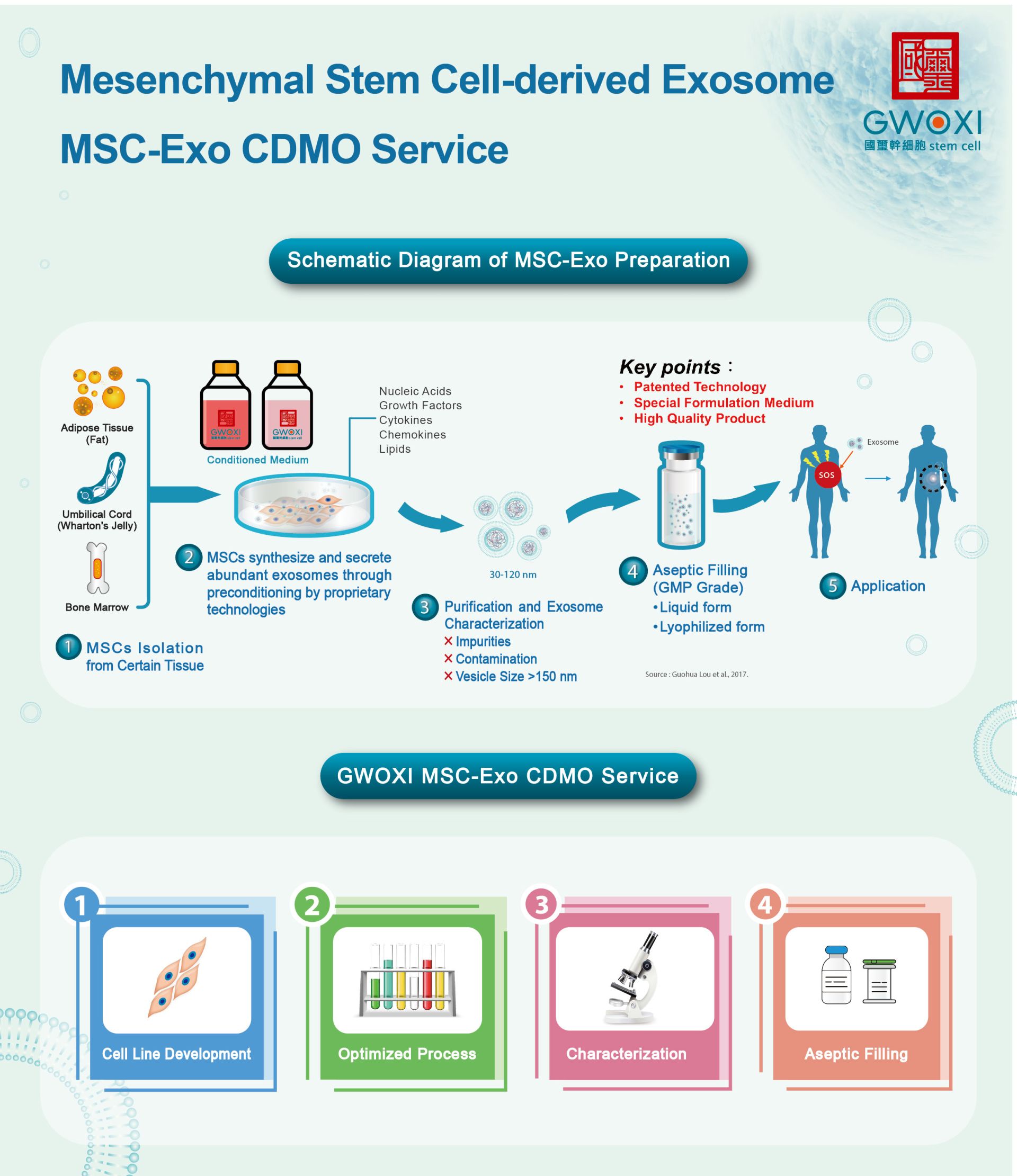
Service Workflow:
- 1. Consultation
Define requirements and intended use. - 2. Agreement
Confirm specification and contract. - 3. Cell Culture, Purification, and Collection
Conduct cell expansion, exosome purification, and exosome collection. - 4. QC Testing
Conduct quality testing to characterize collected exosome. - 5. Shipping and Testing Report
Provide final product attached with comprehensive CoA.
For further inquiries or quotation requests, please feel free to contact us.
Press Releases
Press Releases-
 20202511Press ReleasesGwo Xi Stem Cell Files Drug Master File (DMF) with FDA, Integrating into the Global Regenerative Medicine Industry Chain
20202511Press ReleasesGwo Xi Stem Cell Files Drug Master File (DMF) with FDA, Integrating into the Global Regenerative Medicine Industry Chain -
 22202510Press ReleasesGwo Xi Stem Cell’s Diabetes Cell Therapy Product GXIPC1® Receives Phase I Trial Results Approval from Vietnam’s MOH
22202510Press ReleasesGwo Xi Stem Cell’s Diabetes Cell Therapy Product GXIPC1® Receives Phase I Trial Results Approval from Vietnam’s MOH -
 26202509Press ReleasesGWOXI Stem Cell Secures Taiwan Invention Patent for Stem Cell-Derived Composition in Wound Healing
26202509Press ReleasesGWOXI Stem Cell Secures Taiwan Invention Patent for Stem Cell-Derived Composition in Wound Healing
