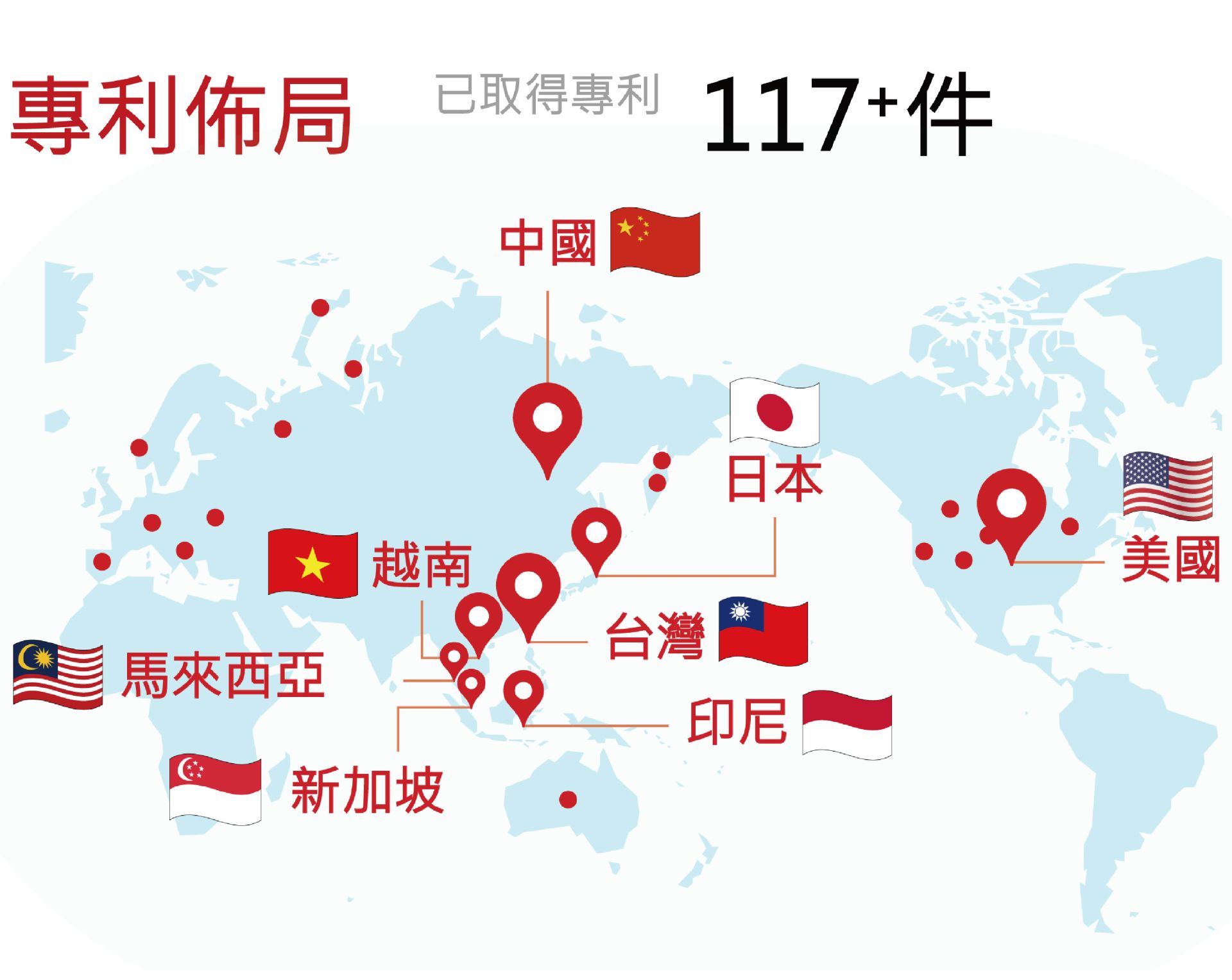
GWOXI Secures More Than 117 Stem Cell Technology Patents to Drive Global Market Expansion.
Taiwan’s cell therapy industry is accelerating the commercialization of new drugs, fueled by breakthroughs in regenerative medicine and the establishment of supportive regulatory frameworks by Taiwanese government. Leveraging its robust patent portfolio and integrated technology capabilities across industry chain, GWOXI (TPEx.: 6704) has successfully advanced multiple innovative technologies from preclinical studies to clinical trials, offering novel therapeutic options for patients.
Patent Portfolio and Technological Innovation: Strengthening Global Competitiveness
Gwo Xi has long been dedicated to the fields of cell therapy and regenerative medicine, securing its technological leadership through a comprehensive global patent strategy. To date, the company has obtained 117 apporved patents worldwide, covering key areas including cell isolation and purification, large-scale production, proprietary conditioned media, exclusive cell processing technologies, automation, formulation, filling, packaging, and international cold-chain delivery. This strengthens its global market competitiveness and attracts international partners to jointly promote the clinical application of cell therapies.
The company has successfully established standardized production processes for mesenchymal stem cells to enhance consistency in cell quality and therapeutic efficacy, and has collected real world data via multiple clinical trials to validate safety and efficacy of the stem cell medicines. Furthermore, by employing AI-assisted analytics and high-throughput screening technologies, Gwo Xi continues to optimize its cell therapy platforms to improve treatment outcomes and reduce costs, laying a solid foundation for future commercialization.

GWOXI’s cell processing facility complies with PIC/S GMP standards, supporting international cell therapy product development.
Industry Integration and Clinical Translation: Dring Accelerated Commercialization
Through a vertically integrated model connecting R&D, manufacturing and clinical applications, Gwo Xi ensures the rapid tranlation of technologies to the market. The company operates a PIC/S GMP-compliant cell processing facility and provides global partners with CDMO (Contract Development and Manufacturing Organization) services for the development and production of cell therapy products. By employing flexible business models—including licensing-out, co-development, and technology transfer—GWOXI accelerates the market entry of stem cell therapy products accross regions while enhancing product value.

GWOXI actively collaborates with international partners to advance technology licensing and co-development, thereby expanding its global market presence.
Global Market Expansion Strategy: Aligning with Market Trends and International Standards
According to Mordor Intelligence, the global cell therapy market value is expected to reach USD 5.58 billion by 2025, with a projected compound annual growth rate (CAGR) of 17.05% from 2025 to 2030, driven by rising prevalence of chronic diseases, growing acceptance of regenerative medicine, and advancements in allogeneic cell technologies.
In line with these market trends, GWOXI actively collaborates with leading international biotechnology companies, medical institutions, and investment funds to strengthen industry integration. Centered on its cell therapy products, PIC/S GMP-compliant facilities, and CDMO services, the company is enhancing alignment with global markets by driving technology licensing and co-development initiatives, providing innovative cell therapy solutions to patients worldwide.
Upholding the principles of “Innovation, Excellence, and Responsibility,” GWOXI continues to advance the commercialization of stem cell medicines. By leveraging its strong patent portfolio and integrated technologies across the industry chain, GWOXI aims to establish an internationally recognized cell therapy brand and collaborate with global partners to pioneer a new era of regenerative medicine.
