Academy
-
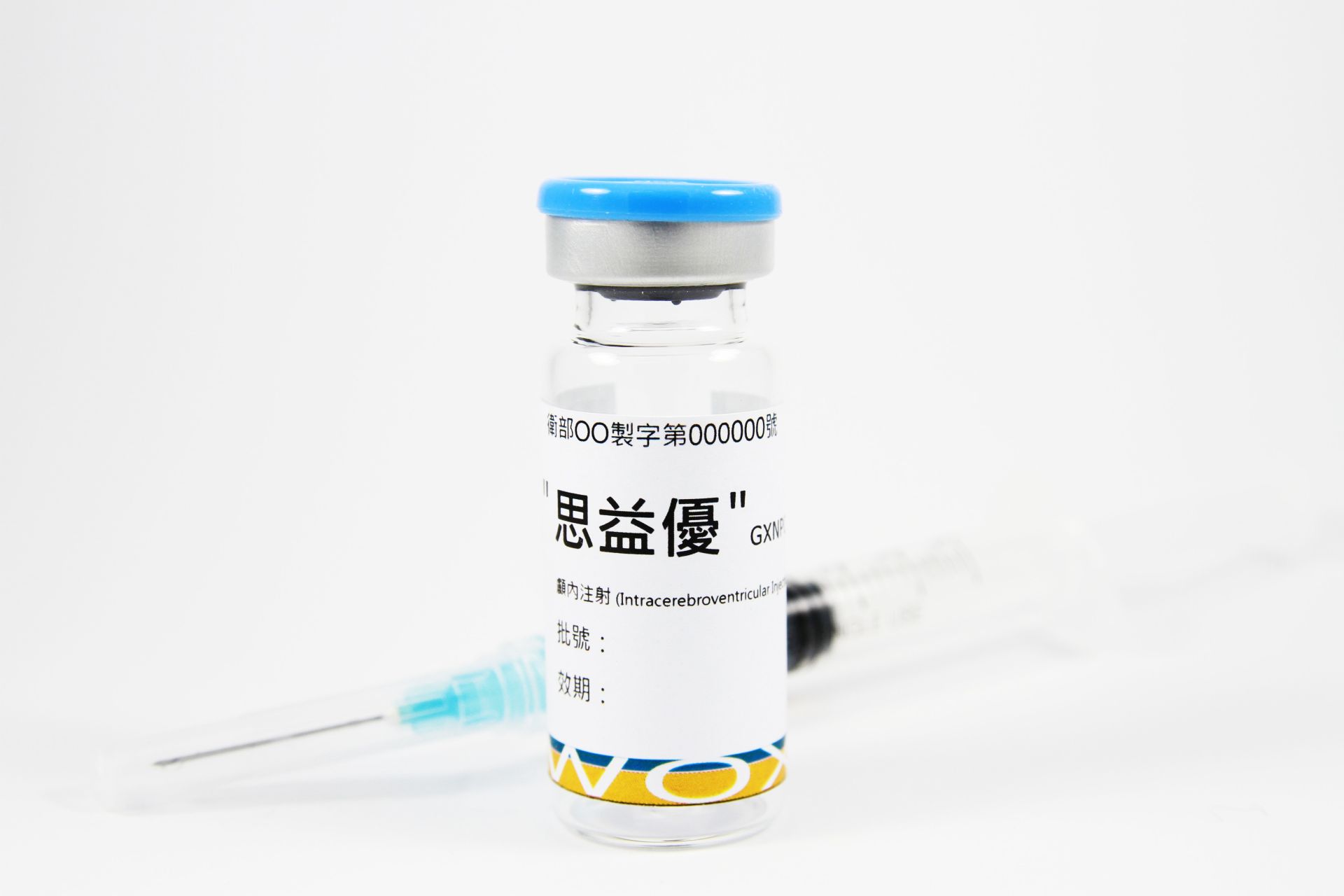 11202507Academy
11202507AcademyGXNPC1® (Chronic Stroke)
GXNPC1® has demonstrated significant neurological improvement in patients with chronic stroke.
-
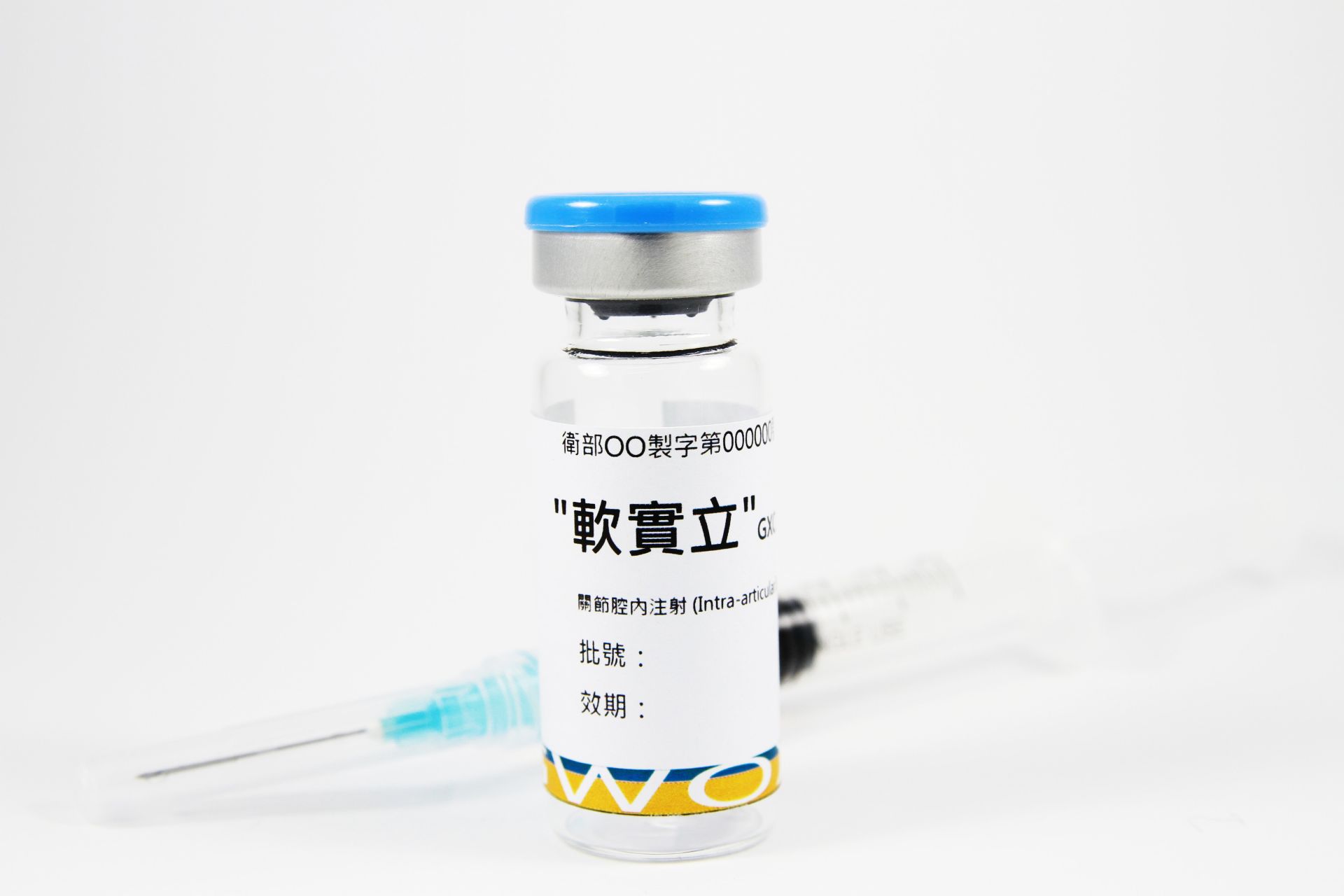 10202507Academy
10202507AcademyGXCPC1® (Knee Osteoarthritis)
This study showed that the intra-articular administration of GXCPC1®, an allogeneic adipose-derived stem cell, was safe and well-tolerated in subjects with therapeutic alternatives to treat knee osteoarthritis (knee OA) during 1 year follow-up period.
-
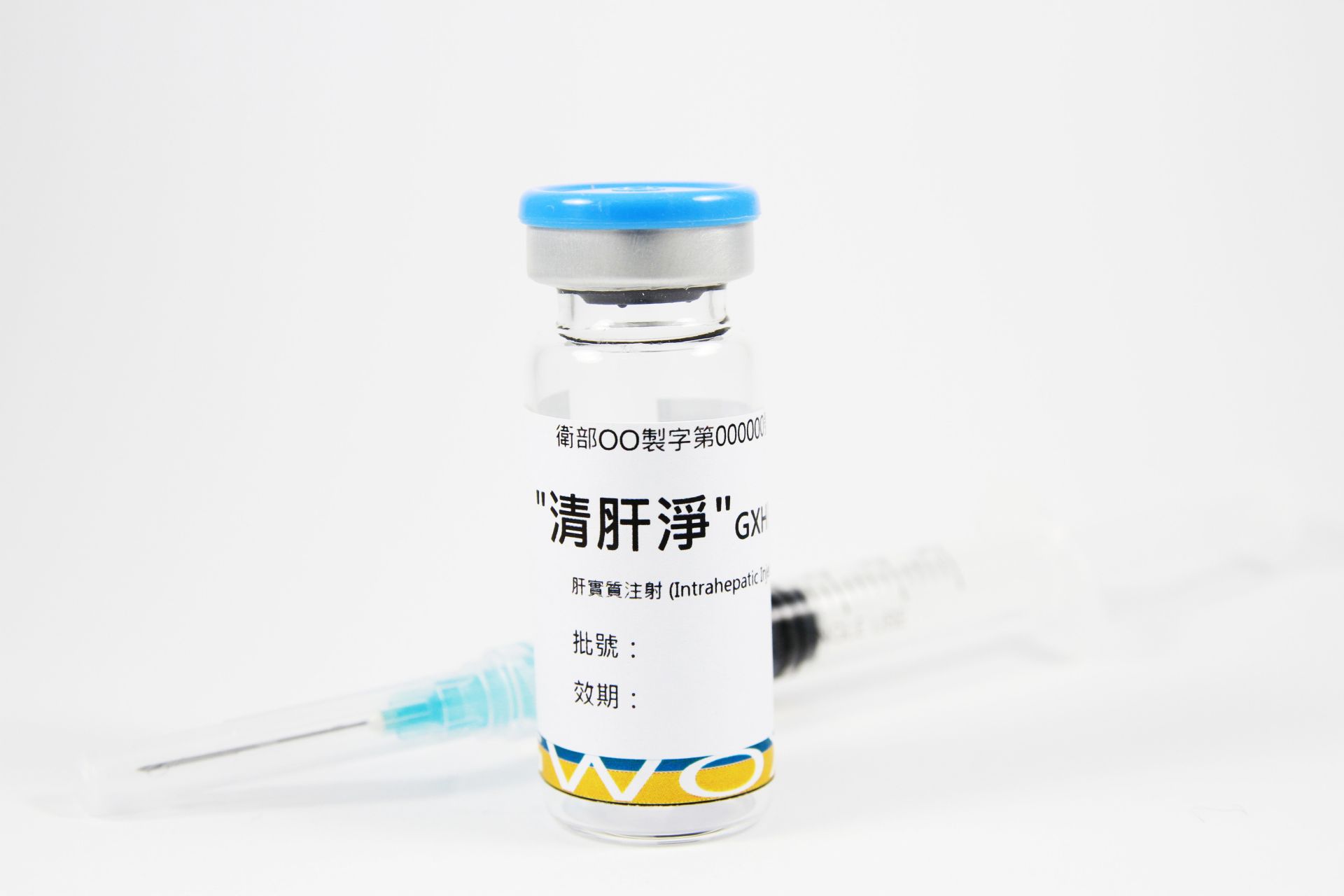 09202507Academy
09202507AcademyGXHPC1® (Liver Cirrhosis)
GXHPC1® demonstrated favorable safety and efficacy in its Phase I human clinical trial. No safety concerns were observed during follow-up period, and patients with liver cirrhosis showed a tendency of improvements for liver function, METAVIR score, Child–Pugh score, MELD score, and quality of life.
-
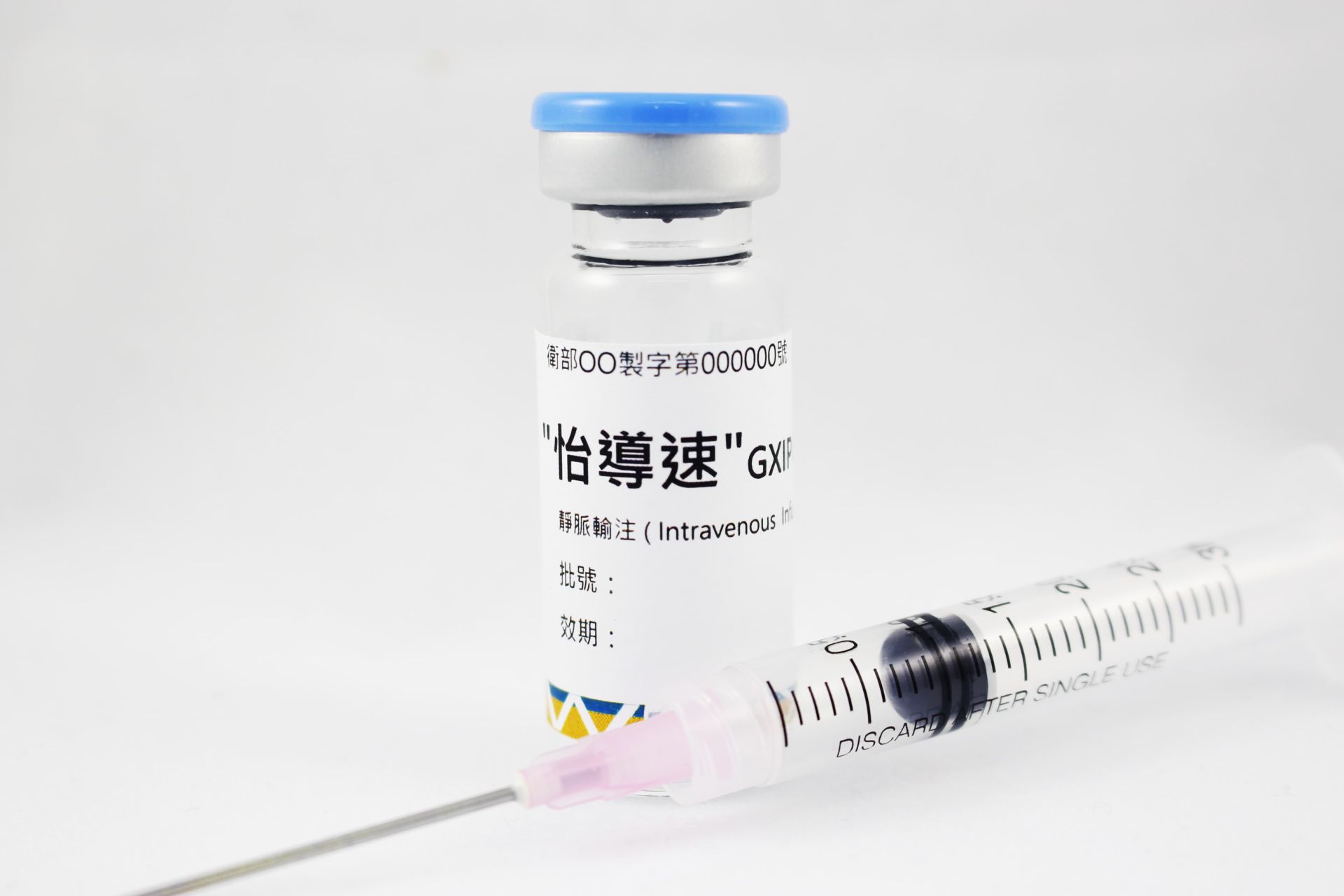 08202507Academy
08202507AcademyGXIPC1® (Type I Diabetes)
The results demonstrated that transplantation of hIPCs significantly alleviates hyperglycemia in streptozotocin-induced diabetic rats, offering a promising approach for safe and cost-effective diabetes treatment.
-
 07202507Academy
07202507AcademyGX Factor® (Growth Factor)
PRFr (GX Factor®) releases abundant of growth factors such as PDGF-AB, IGF-1, and TGF-β1 within 300 minutes, suggesting that GX Factor® can effectively enhance growth factor release and promote wound healing.
-
 07202507Academy
07202507AcademyThe Application of Cardiovascular Disease
In this study, it showed that BP-primed human adipose-derived stem cells (BP-pretreated hADSCs) significantly reduced arrhythmias following myocardial infarction and enhanced the cells’ cardioprotective effects via the PI3K/Akt/GSK-3β pathway.
