相本
-
 11202602Publications
11202602PublicationsWhat are Extracellular Vesicles (EVs)? Sub
Simply stated, EVs can be regarded as "small delivery parcels" that facilitate intercellular communication; they comprise various apoptotic bodies, microvesicles, and exosomes.
-
 09202602Publications
09202602PublicationsWhat Are the Different Sources of Exosomes? Animal
Explore animal and plant-derived exosome sources , highlighting their distinct extraction methods and diverse applications.
-
 09202602Publications
09202602PublicationsWhat Are Growth Factors? Revealing the Truth Behin
Explore growth factors and their three major applications in regenerative medicine, skin repair, and clinical diagnostics.
-
 23202512Publications
23202512PublicationsGwo Xi Stem Cell’s Novel Stroke Therapy GXNPC1 Pub
Gwo Xi Stem Cell’s novel stem cell therapy for stroke, GXNPC1®, has had its latest research findings published in the international academic journal The ACTO Times (Vol. 2, Issue 4, Autumn 2025) under the title “Gwo Xi’s stem cell product GXNPC1 shows safety and beneficial effect for stroke patients.”
-
 20202511Brochures
20202511BrochuresClinical-Grade Stem Cell as Starting Material (
-
 03202511Publications
03202511PublicationsStem Cell Therapy: Autologous vs. Allogeneic Appro
In clinical practice, Stem Cell Therapy (also known as Regenerative Medicine) involves a treatment process where cells are professionally processed prior to administration. The stem cells must be harvested from the human body and undergo rigorous processing, such as purification, isolation, culture, and quality testing. This meticulous processing ensures the quality of the final cell-based product before it is administered into the patient's body to repair damaged tissues, modulate the immune system, and promote the regeneration of organs and tissues.
-
 27202510Publications
27202510PublicationsHow Do EXOSOMES Work in Aesthetic Medicine: Unlock
In recent years, regenerative medicine has progressed alot. After unveiling stem cells’ regenerative potential, scientists began to explore the beneficial effects of exosome in the field of organ/tissue repair and anti-aging. Because stem cell therapies are costly and risky for the reasons like self-copy and tumorigenesis, exosomes secreted by stem cells, with high safety and biocompatibility profiles, have become a key bridge between regenerative medicine and various industries. Exosomes are constantly discussed and explored in the realm of aesthetic skincare.
-
 21202510Publications
21202510PublicationsWhat Are Exosomes? Decoding Stem Cell Exosome Prop
Exosomes are nanoscale extracellular vesicles (EVs) secreted by cells, ranging in size from 30 to 150 nanometers (nm). They are commonly found in bodily fluids such as blood, urine, saliva, and others.
-
 17202510Publications
17202510PublicationsExosome Hair Growth Therapy: Say Goodbye to Male P
Exosome-based hair regrowth takes a different approach. It is minimally invasive and combines tissue repair with follicle activation.
-
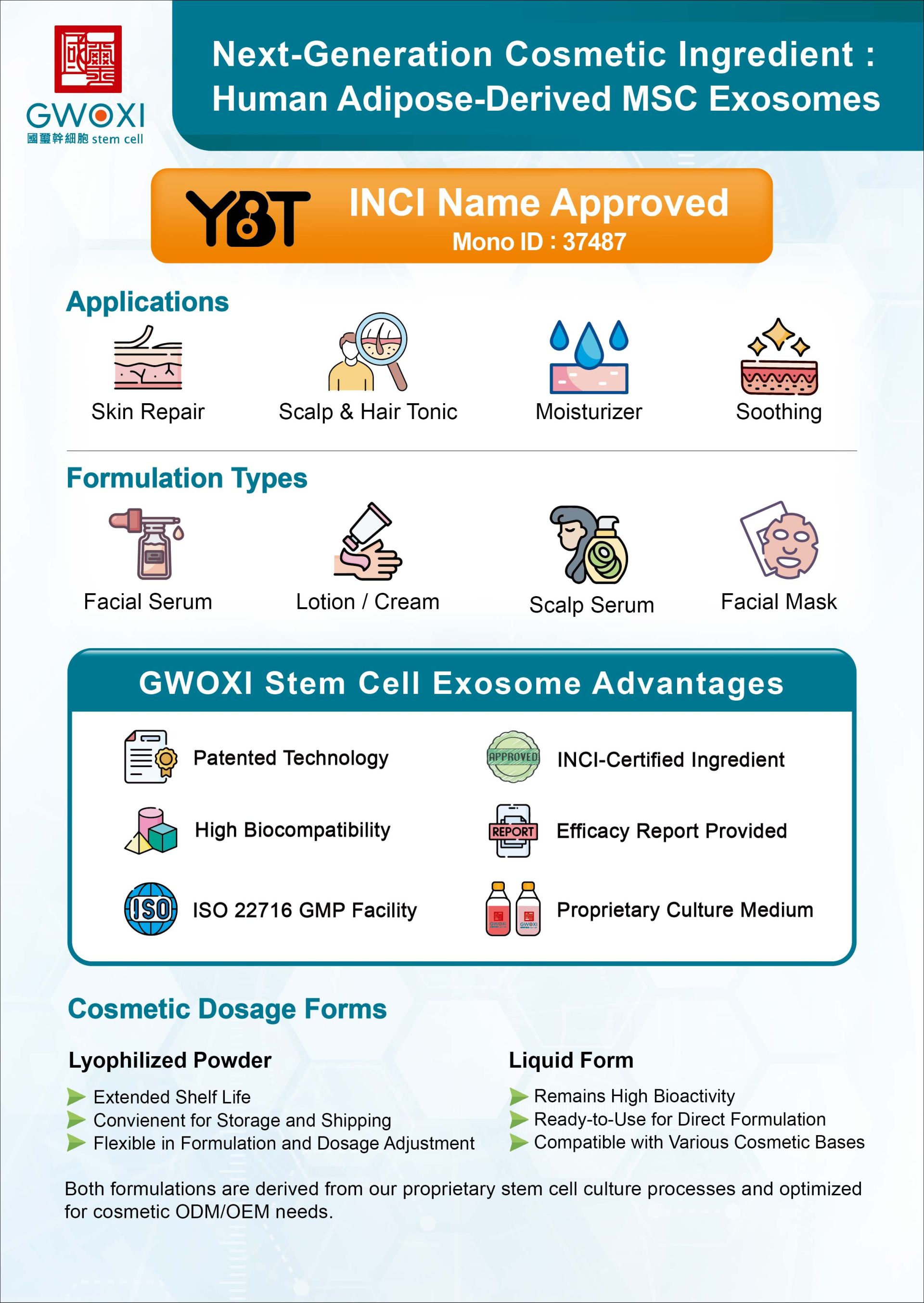 05202508Brochures
05202508BrochuresCosmetic Ingredient: hMSC-Exos
-
 05202508Brochures
05202508BrochuresCompany Profile
-
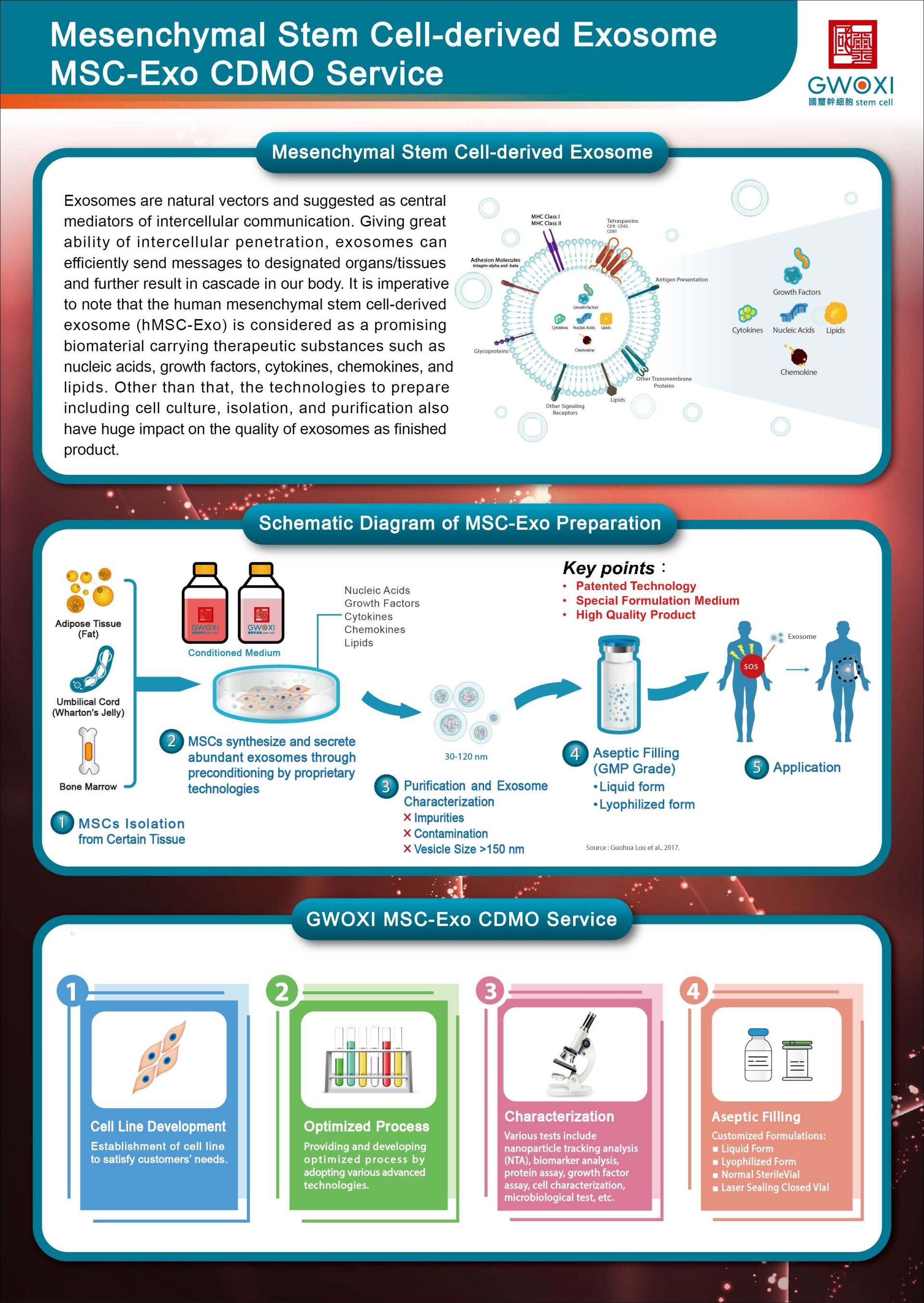 05202508Brochures
05202508BrochuresMSC-Exo CDMO Service
-
 21202507Publications
21202507PublicationsWhat Is Licensing Transaction in Pharmaceutical In
Licensing Transactions are a common collaboration model in the biotech and pharmaceutical industry.
-
 11202507Academy
11202507AcademyGXNPC1® (Chronic Stroke)
GXNPC1® has demonstrated significant neurological improvement in patients with chronic stroke.
-
 10202507Academy
10202507AcademyGXCPC1® (Knee Osteoarthritis)
This study showed that the intra-articular administration of GXCPC1®, an allogeneic adipose-derived stem cell, was safe and well-tolerated in subjects with therapeutic alternatives to treat knee osteoarthritis (knee OA) during 1 year follow-up period.
-
 09202507Academy
09202507AcademyGXHPC1® (Liver Cirrhosis)
GXHPC1® demonstrated favorable safety and efficacy in its Phase I human clinical trial. No safety concerns were observed during follow-up period, and patients with liver cirrhosis showed a tendency of improvements for liver function, METAVIR score, Child–Pugh score, MELD score, and quality of life.
-
 08202507Academy
08202507AcademyGXIPC1® (Type I Diabetes)
The results demonstrated that transplantation of hIPCs significantly alleviates hyperglycemia in streptozotocin-induced diabetic rats, offering a promising approach for safe and cost-effective diabetes treatment.
-
 07202507Academy
07202507AcademyGX Factor® (Growth Factor)
PRFr (GX Factor®) releases abundant of growth factors such as PDGF-AB, IGF-1, and TGF-β1 within 300 minutes, suggesting that GX Factor® can effectively enhance growth factor release and promote wound healing.
-
 07202507Academy
07202507AcademyThe Application of Cardiovascular Disease
In this study, it showed that BP-primed human adipose-derived stem cells (BP-pretreated hADSCs) significantly reduced arrhythmias following myocardial infarction and enhanced the cells’ cardioprotective effects via the PI3K/Akt/GSK-3β pathway.
-
 25202504Publications
25202504PublicationsWhat Makes Stem Cell Medicines Different from Trad
With advances in medical science, the ways we treat disease are continuously evolving.
